Citation: Babcock and Booth (2020) Monitoring Methods. Tern Conservation Best Practice. Produced for “Improving the conservation prospects of the priority species roseate tern throughout its range in the UK and Ireland” LIFE14 NAT/UK/000394’
Last updated: October 2020
This is a live document we update regularly. If you have comments and suggestions, please email Chantal.Macleod-Nolan@rspb.org.uk
Last updated: October 2020
This is a live document we update regularly. If you have comments and suggestions, please email Chantal.Macleod-Nolan@rspb.org.uk
| Babcock and Booth (2020) Monitoring Methods. Tern Conservation Best Practice. | |
| File Size: | 1959 kb |
| File Type: | |
Key Messages
- Monitoring is a key part of tern colony management, helping to identify any issues, determine which management interventions are effective, and contributing to the national dataset via the Seabird Monitoring Programme.
- Using standardised monitoring methods adds greatly to the value of these data by ensuring comparisons can be made between years and between colonies. However, if there is a long run e.g. 10 years or more, of data collected to consistent but non-standard methods, this also has value and careful consideration should be given before changing monitoring methods.
- If monitoring methods are changed, a period of using both methods, if possible, will allow the data from the two methods to be calibrated, and ensure some level of continuity in the dataset.
- On seabird islands, biosecurity planning and associated monitoring are key elements of management
Information
Accurate information on seabird breeding numbers, population changes and breeding success informs conservation management and conservation policy. Without this data it is impossible to understand what is happening in a colony and whether conservation management strategies have been successful or not. Standardising the methods used collecting in the data enables meaningful comparisons to be made between years and between colonies.
Synchronous monitoring is particularly valuable for tern species since large numbers of birds, or even a whole colony, can move to a different location between years or occasionally within the breeding season, particularly if there has been disturbance or heavy predation. Often such birds reappear at another site, for example the Sandwich terns Thalasseus sandvicensis from Cemlyn Bay, North Wales which relocated to Hodbarrow in Northwest England in the face of predation pressure in 2018. For all large tern colonies annual monitoring is appropriate, and in the national seabird census efforts were made to count birds at all the tern colonies of all sizes in a single year to minimise the chances of double-counting or missing birds which relocated.
In the UK and Ireland, methods for monitoring population size, population changes and productivity for most resident breeding seabird species are included in Walsh et. al. (1995) the Seabird Monitoring Handbook for Britain and Ireland, which is available from the JNCC Seabird Monitoring Programme website.
The Seabird Monitoring Handbook provides a range of population and productivity monitoring methods, which aim to cover different circumstances, accepting that there may be a compromise between accuracy and practicality. The Handbook is periodically reviewed, so these methods will not remain unchanged over time and should in future adapt to include possibilities such as using drones, cameras and artificial intelligence.
The methods in the Seabird Monitoring Handbook will not be duplicated here. However, monitoring methods for other useful parameters such as adult survival rates, or chick provisioning, which are not covered by the Handbook are included.
Monitoring Principles
Biosecurity
Biosecurity measures and the associated monitoring are an extremely important part of managing a seabird island. Introduced predators have caused catastrophic damage to many species, including numerous extirpations as well as contributing to ongoing declines to island species around the world. Many of the UK’s island ecosystems have been damaged by the arrival and establishment of invasive non-native species, and ground-nesting species such as terns are particularly vulnerable to invasive predators. The UK Rodent Eradication Best Practice Toolkit provides guidance and templates for biosecurity planning and rodent surveillance as well as eradications. This toolkit is based on the New Zealand Department of Conservation’s ‘Best Practice for rat eradication – bait station’ and the Pacific Invasives Initiative Resource Kits, adapted for use in the UK legislative context. The focus of the toolkit is invasive rodents, but biosecurity measures to protect seabird islands against other species (e.g. American mink, stoats, weasels, hedgehogs, feral cats) is also likely to be required and should be included in biosecurity planning, following similar principles but adapting methods to take account of the ecology of the likely threat species. Planning and monitoring is key to effective biosecurity, and all important seabird islands should have a biosecurity plan and monitoring in place, together with an incursion response plan. Every seabird island is different and so these should be individually tailored to the unique combination of geography, important species and threats. Mainland sites or those with native mammalian predators are likely to require protective measures such as anti-predator fencing.
Accurate information on seabird breeding numbers, population changes and breeding success informs conservation management and conservation policy. Without this data it is impossible to understand what is happening in a colony and whether conservation management strategies have been successful or not. Standardising the methods used collecting in the data enables meaningful comparisons to be made between years and between colonies.
Synchronous monitoring is particularly valuable for tern species since large numbers of birds, or even a whole colony, can move to a different location between years or occasionally within the breeding season, particularly if there has been disturbance or heavy predation. Often such birds reappear at another site, for example the Sandwich terns Thalasseus sandvicensis from Cemlyn Bay, North Wales which relocated to Hodbarrow in Northwest England in the face of predation pressure in 2018. For all large tern colonies annual monitoring is appropriate, and in the national seabird census efforts were made to count birds at all the tern colonies of all sizes in a single year to minimise the chances of double-counting or missing birds which relocated.
In the UK and Ireland, methods for monitoring population size, population changes and productivity for most resident breeding seabird species are included in Walsh et. al. (1995) the Seabird Monitoring Handbook for Britain and Ireland, which is available from the JNCC Seabird Monitoring Programme website.
The Seabird Monitoring Handbook provides a range of population and productivity monitoring methods, which aim to cover different circumstances, accepting that there may be a compromise between accuracy and practicality. The Handbook is periodically reviewed, so these methods will not remain unchanged over time and should in future adapt to include possibilities such as using drones, cameras and artificial intelligence.
The methods in the Seabird Monitoring Handbook will not be duplicated here. However, monitoring methods for other useful parameters such as adult survival rates, or chick provisioning, which are not covered by the Handbook are included.
Monitoring Principles
- All monitoring activity which involves approaching breeding terns must be done in compliance with all applicable local laws and licensing requirements. Colony managers should get specific advice on this before commencing any activity in a colony.
- Monitoring should not be conducted during heavy rain, strong winds or wet weather, to minimise the risk of eggs or chicks becoming chilled. Visits in very hot conditions should also be avoided. This is particularly the case when chicks are small and require brooding to keep warm or shade to keep cool.
- Where monitoring methods involve entering a colony or disturbing birds, the time spent should be kept to a minimum, and where this is longer than is a reasonable amount of time to keep birds from their nest and chicks (adjusted for weather conditions), monitoring should be split over multiple sessions with a ‘recovery period’ in between to allow birds to feed and brood.
Biosecurity
Biosecurity measures and the associated monitoring are an extremely important part of managing a seabird island. Introduced predators have caused catastrophic damage to many species, including numerous extirpations as well as contributing to ongoing declines to island species around the world. Many of the UK’s island ecosystems have been damaged by the arrival and establishment of invasive non-native species, and ground-nesting species such as terns are particularly vulnerable to invasive predators. The UK Rodent Eradication Best Practice Toolkit provides guidance and templates for biosecurity planning and rodent surveillance as well as eradications. This toolkit is based on the New Zealand Department of Conservation’s ‘Best Practice for rat eradication – bait station’ and the Pacific Invasives Initiative Resource Kits, adapted for use in the UK legislative context. The focus of the toolkit is invasive rodents, but biosecurity measures to protect seabird islands against other species (e.g. American mink, stoats, weasels, hedgehogs, feral cats) is also likely to be required and should be included in biosecurity planning, following similar principles but adapting methods to take account of the ecology of the likely threat species. Planning and monitoring is key to effective biosecurity, and all important seabird islands should have a biosecurity plan and monitoring in place, together with an incursion response plan. Every seabird island is different and so these should be individually tailored to the unique combination of geography, important species and threats. Mainland sites or those with native mammalian predators are likely to require protective measures such as anti-predator fencing.
Case Studies
Population and productivity monitoring: a minimum standard for the South Coast Tern Network
The following methodology was developed for the South Coast Tern Network. It was proposed as a minimum standard to gather basic demography metrics across the regional metapopulation. This method does assume that multiple visits to the colony will be possible; if this is not the case and the objective is to get a one-off count of a remote colony then Seabird Monitoring Methods Handbook Census method 3 (flush counts of individual adults) should be used.
As a minimum, three metrics should be collected every year at each colony:
A minimum of three visits are required to determine the beginning of the incubation period, count AONs and assess productivity.
First egg date: this information is useful to determine suitable dates for the counts of AONs and large chicks later in the season. The developing colony should be observed from a vantage point as birds are vulnerable to disturbance in this period. The more visits that are undertaken, the more precise the first egg date, which should be established separately for each colony or sub-colony.
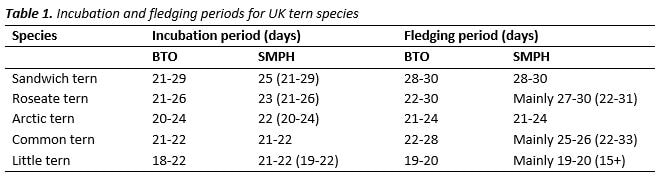
Count of AONs - Visual counts: Sites with little vegetation and flat topography can allow all or the majority of birds to be monitored by a visual count from a distance. Larger and less visible colonies should be surveyed by entering the colony (see below). Visual counts can be carried out as many times of as possible from mid-May to late June, but as a minimum, just before hatching of first chicks (3 to 3.5 weeks from the first egg date). Average incubation periods for each species are given in the Seabird Monitoring Handbook and on the BTO BirdFacts pages and for UK tern species are summarised in the table below.
Count only birds which appear to be sitting on a nest. With little practice incubating birds can be distinguished from resting birds by a different posture, i.e. lower position and higher raised tail. Note that some of these birds can be hidden by vegetation so several counts from different vantage points can yield different numbers.
Report: the peak count of AONs should be used as the definitive figure (but the numbers of AONs for each visit and their dates should also reported).
Population and productivity monitoring: a minimum standard for the South Coast Tern Network
The following methodology was developed for the South Coast Tern Network. It was proposed as a minimum standard to gather basic demography metrics across the regional metapopulation. This method does assume that multiple visits to the colony will be possible; if this is not the case and the objective is to get a one-off count of a remote colony then Seabird Monitoring Methods Handbook Census method 3 (flush counts of individual adults) should be used.
As a minimum, three metrics should be collected every year at each colony:
- Peak number of Apparently Occupied Nests (AONs)
- Average clutch size (for colonies where walk through surveys are carried out)
- Number of large and fledged chicks
A minimum of three visits are required to determine the beginning of the incubation period, count AONs and assess productivity.
First egg date: this information is useful to determine suitable dates for the counts of AONs and large chicks later in the season. The developing colony should be observed from a vantage point as birds are vulnerable to disturbance in this period. The more visits that are undertaken, the more precise the first egg date, which should be established separately for each colony or sub-colony.
Count of AONs - Visual counts: Sites with little vegetation and flat topography can allow all or the majority of birds to be monitored by a visual count from a distance. Larger and less visible colonies should be surveyed by entering the colony (see below). Visual counts can be carried out as many times of as possible from mid-May to late June, but as a minimum, just before hatching of first chicks (3 to 3.5 weeks from the first egg date). Average incubation periods for each species are given in the Seabird Monitoring Handbook and on the BTO BirdFacts pages and for UK tern species are summarised in the table below.
Count only birds which appear to be sitting on a nest. With little practice incubating birds can be distinguished from resting birds by a different posture, i.e. lower position and higher raised tail. Note that some of these birds can be hidden by vegetation so several counts from different vantage points can yield different numbers.
Report: the peak count of AONs should be used as the definitive figure (but the numbers of AONs for each visit and their dates should also reported).
In larger or less visible colonies, in-colony nest counts should be used. The frequency of visits should be limited to once a week and carried out only in good weather and for a maximum of 20 minutes (multiple surveyors can work simultaneously to minimise the time spent in the colony). The survey should be conducted 3-3.5 weeks from the first egg date, but if many nests are found to contain incomplete clutches repeat the count a week later. Record clutch sizes for each nest plus any predated nests and eggs.
If possible, repeat the count once a week after the initial visit up to the end of June.
Report: peak count of active nests with eggs for the whole colony, and average clutch size
Very large colonies can be surveyed using the ‘pasta method’ to allow rapid counting of the population plus a sample for clutch size, 3-3.5 weeks from the first egg date. This will require several people and may also need to be done in sections to minimise the disturbance time to the colony. Prepare a known number of bags each containing 90 pieces of normal dried pasta plus 10 pieces of a different shape or colour. Surveyors walk through the colony in a line a short distance apart, placing a pasta piece next to each nest. When a coloured or different shaped piece of pasta is used, record clutch size (this will give a random sample of clutch size for 10% of the nests). When all the nests have been included, count the number of empty bags and the number of pasta pieces left in any partially used bags.
Report: the number of AON (the number of empty pasta bags multiplied by 100, with the number of pasta pieces remaining subtracted from the total).
The average clutch size (the number of eggs from every nest with coloured/different-shaped pasta, divided by the number of nests for which clutch size was recorded).
Productivity Monitoring: based on the Seabird Monitoring Handbook Tern productivity monitoring method 4 (nest/incubating adult count, with single count of large chicks).
At smaller or visible colonies, all chicks may be visible through a telescope. Once significant numbers of large chicks are present in the colony count the number of fledged chicks as often as possible. Provided all the fledged birds originate from the colony (rather than being on passage from other colonies), use the peak number of fledged birds. Estimate productivity by dividing the peak number of fledged chicks by the peak number of AONs. This is likely to be an under-estimate especially if the colony is not synchronous.
For larger colonies, estimate when the first chicks are likely to fledge from the date of first egg/ start of incubation period or early hatched chicks (see table above). Walk through the colony and count the number of large (10-14 days old, depending on the species) and the number of fledged chicks on a single day. Keep a separate count of smaller chicks and unhatched eggs.
Estimate annual productivity as the number of large (10-14 days old) and fledged chicks recorded during the visit, divided by the peak number of AONs. Once tern chicks are a few days old they can be quite mobile, and difficult to find, particularly in thick vegetation. As a result, this method may produce a substantial underestimate of productivity in some cases (as some large chicks may be missed, while smaller/unhatched chicks may also survive), especially if the colony is asynchronous.
If possible, repeat the count once a week after the initial visit up to the end of June.
Report: peak count of active nests with eggs for the whole colony, and average clutch size
Very large colonies can be surveyed using the ‘pasta method’ to allow rapid counting of the population plus a sample for clutch size, 3-3.5 weeks from the first egg date. This will require several people and may also need to be done in sections to minimise the disturbance time to the colony. Prepare a known number of bags each containing 90 pieces of normal dried pasta plus 10 pieces of a different shape or colour. Surveyors walk through the colony in a line a short distance apart, placing a pasta piece next to each nest. When a coloured or different shaped piece of pasta is used, record clutch size (this will give a random sample of clutch size for 10% of the nests). When all the nests have been included, count the number of empty bags and the number of pasta pieces left in any partially used bags.
Report: the number of AON (the number of empty pasta bags multiplied by 100, with the number of pasta pieces remaining subtracted from the total).
The average clutch size (the number of eggs from every nest with coloured/different-shaped pasta, divided by the number of nests for which clutch size was recorded).
Productivity Monitoring: based on the Seabird Monitoring Handbook Tern productivity monitoring method 4 (nest/incubating adult count, with single count of large chicks).
At smaller or visible colonies, all chicks may be visible through a telescope. Once significant numbers of large chicks are present in the colony count the number of fledged chicks as often as possible. Provided all the fledged birds originate from the colony (rather than being on passage from other colonies), use the peak number of fledged birds. Estimate productivity by dividing the peak number of fledged chicks by the peak number of AONs. This is likely to be an under-estimate especially if the colony is not synchronous.
For larger colonies, estimate when the first chicks are likely to fledge from the date of first egg/ start of incubation period or early hatched chicks (see table above). Walk through the colony and count the number of large (10-14 days old, depending on the species) and the number of fledged chicks on a single day. Keep a separate count of smaller chicks and unhatched eggs.
Estimate annual productivity as the number of large (10-14 days old) and fledged chicks recorded during the visit, divided by the peak number of AONs. Once tern chicks are a few days old they can be quite mobile, and difficult to find, particularly in thick vegetation. As a result, this method may produce a substantial underestimate of productivity in some cases (as some large chicks may be missed, while smaller/unhatched chicks may also survive), especially if the colony is asynchronous.
Coquet Island: productivity monitoring with enclosures for common and Arctic tern
An alternative method for monitoring productivity is to enclose a sample of nests so that chicks can be re-found until they fledge. If the nests enclosed are representative of the colony as a whole and the sample size is large enough this can be an accurate method, however unrepresentative nests (e.g. early nests in the core of the colony which are likely to belong to experienced adults of good quality) or small sample sizes can introduce significant biases. If there is any reason to suspect that the enclosed nests are not representative of the colony this can be reported along with the results.
Enclosures are low fences around nests or groups of nests constructed from short plastic netting, or chicken wire, supported by short metal stakes or bamboo canes. On Coquet Island groups of one to five nests are enclosed, a total of 30 each of Arctic Sterna paradisaea and common tern Sterna hirundo nests (this is a small sample and should be considered a minimum number). At the Skerries and Rockabill larger groups of nests are enclosed, but the objective is that all the chicks in the enclosure can be safely located without double counting.
Enclosures must be erected carefully with sufficient space around the nests is essential to ensure adults have landing areas (often they have a preferred landing spot near the nest which must be included to ensure they can still reach the nest, as they are not very adaptable in this regard). After erecting the fence, the nests should be monitored from a distance to ensure the adults can return to the nests, it may be necessary to adjust the fence position. The base of the netting should be pinned to the ground, ensuring chicks cannot escape or get caught. Chick shelters should be provided within the enclosures, especially as the enclosure may restrict access to vegetation cover.
Enclosures should be visited, and all nests, eggs, chicks and dead chicks recorded as often as practicable and at least every three to four days. If there is a suitably licenced and experienced ringer in the team, chicks may be ringed to help keep track of progress. When chicks are getting large note of numbers of near-fledging birds, chicks which are seen at 15 days old, can be assumed to fledge (usually at around 25-30 days old).
Report: Productivity, calculated by dividing the number of chicks thought to have fledged by the number of enclosed nests. Check the figure by deducting the number of failed eggs and dead or predated chicks from the number of eggs recorded in each enclosure.
Vegetation management may be required, but if this is done within the breeding season this must be done by hand and with extreme care. The enclosures should also be checked regularly for trapped birds; blown in litter etc.
This method is generally not suitable for Sandwich terns, as they nest at high densities and there is no space do deploy the fencing between the nests. It is also not required for little terns as the chicks are easily visible in sparse vegetation of their preferred habitat.
An alternative method for monitoring productivity is to enclose a sample of nests so that chicks can be re-found until they fledge. If the nests enclosed are representative of the colony as a whole and the sample size is large enough this can be an accurate method, however unrepresentative nests (e.g. early nests in the core of the colony which are likely to belong to experienced adults of good quality) or small sample sizes can introduce significant biases. If there is any reason to suspect that the enclosed nests are not representative of the colony this can be reported along with the results.
Enclosures are low fences around nests or groups of nests constructed from short plastic netting, or chicken wire, supported by short metal stakes or bamboo canes. On Coquet Island groups of one to five nests are enclosed, a total of 30 each of Arctic Sterna paradisaea and common tern Sterna hirundo nests (this is a small sample and should be considered a minimum number). At the Skerries and Rockabill larger groups of nests are enclosed, but the objective is that all the chicks in the enclosure can be safely located without double counting.
Enclosures must be erected carefully with sufficient space around the nests is essential to ensure adults have landing areas (often they have a preferred landing spot near the nest which must be included to ensure they can still reach the nest, as they are not very adaptable in this regard). After erecting the fence, the nests should be monitored from a distance to ensure the adults can return to the nests, it may be necessary to adjust the fence position. The base of the netting should be pinned to the ground, ensuring chicks cannot escape or get caught. Chick shelters should be provided within the enclosures, especially as the enclosure may restrict access to vegetation cover.
Enclosures should be visited, and all nests, eggs, chicks and dead chicks recorded as often as practicable and at least every three to four days. If there is a suitably licenced and experienced ringer in the team, chicks may be ringed to help keep track of progress. When chicks are getting large note of numbers of near-fledging birds, chicks which are seen at 15 days old, can be assumed to fledge (usually at around 25-30 days old).
Report: Productivity, calculated by dividing the number of chicks thought to have fledged by the number of enclosed nests. Check the figure by deducting the number of failed eggs and dead or predated chicks from the number of eggs recorded in each enclosure.
Vegetation management may be required, but if this is done within the breeding season this must be done by hand and with extreme care. The enclosures should also be checked regularly for trapped birds; blown in litter etc.
This method is generally not suitable for Sandwich terns, as they nest at high densities and there is no space do deploy the fencing between the nests. It is also not required for little terns as the chicks are easily visible in sparse vegetation of their preferred habitat.
Monitoring terns using a drone: a trial at Langstone Harbour
The islands in Langstone Harbour are low, narrow, humped shingle forms with scattered vegetation. Monitoring of the mixed tern and gull colony has historically been by boat, including through photography, as landing causes significant disturbance, however parts of the islands cannot be seen by this method due to the shape and lack of vantage points, and wave motion during observations can add to the difficulty.
Trials of tern and gull monitoring by drone took place during 2019, using a Mavic 2 Pro Unmanned Aerial Vehicle (drone), made available from the LIFE Roseate Tern project. Permission was sought and gained from Natural England and the Langstone Harbour Board pre-season and the Site Manager received drone training.
Four flights were undertaken on the reserve, two of these were over Long Island (away from the main colony) with the other two over South Binness (the core colony) on 24th and 25th June.
On the first attempt on Long Island, the drone drew almost immediate attention from Oystercatchers Haematopus ostralegus with up to 10 at a time flying in large circles around it during most of the flight. Other birds did not react with the exception of those within the immediate launch and landing area. The footage gained was of a superb quality and the whole process proceeded very smoothly with the whole flight lasting approximately 10 minutes.
The second trial flight was over South Binness, the core of the colony, and an initial approach was made at a height of c. 50m resulting in birds in a large part of the nearby colony taking flight in a ‘dread’. Forward motion was stopped, and the drone was raised to height of c. 80m while the birds resettled. Forward flight was resumed at 80m and no further disturbance was observed. The photos were subsequently analysed and although a full count of sitting birds would be possible, the relatively high position of the drone during most of the flight meant that differentiation of species and nesting/roosting birds was difficult.
On the third and fourth trials the drone was launched and bought up to an altitude of 80m, moved into position then then slowly lowered, watching carefully for signs of disturbance. Over the course of 90 seconds the drone was lowered to 15m without any disturbance response. Photos were taken on the ‘auto’ setting and later analysed by zooming in on generic software (Microsoft Paint 3D) and the young of each species were counted. These results were then compared to the most recent standard boat-based productivity survey which had taken place just two days earlier. The drone survey revealed a surprising 64% increase in the number of black-headed gull Chroicocephalus ridibundus young recorded.
The analysis of the images via the method above took considerable time however and as a result (and due to the many other pressures on time during the breeding seabird season) further flights for the purpose of monitoring the rest of the colony did not take place during 2019. There are faster ways of analysing the images but these require some specialist software. It is also worth considering the possibility of engaging citizen scientists to analyse images e.g. SeabirdWatch.
Key Points when using drones
The islands in Langstone Harbour are low, narrow, humped shingle forms with scattered vegetation. Monitoring of the mixed tern and gull colony has historically been by boat, including through photography, as landing causes significant disturbance, however parts of the islands cannot be seen by this method due to the shape and lack of vantage points, and wave motion during observations can add to the difficulty.
Trials of tern and gull monitoring by drone took place during 2019, using a Mavic 2 Pro Unmanned Aerial Vehicle (drone), made available from the LIFE Roseate Tern project. Permission was sought and gained from Natural England and the Langstone Harbour Board pre-season and the Site Manager received drone training.
Four flights were undertaken on the reserve, two of these were over Long Island (away from the main colony) with the other two over South Binness (the core colony) on 24th and 25th June.
On the first attempt on Long Island, the drone drew almost immediate attention from Oystercatchers Haematopus ostralegus with up to 10 at a time flying in large circles around it during most of the flight. Other birds did not react with the exception of those within the immediate launch and landing area. The footage gained was of a superb quality and the whole process proceeded very smoothly with the whole flight lasting approximately 10 minutes.
The second trial flight was over South Binness, the core of the colony, and an initial approach was made at a height of c. 50m resulting in birds in a large part of the nearby colony taking flight in a ‘dread’. Forward motion was stopped, and the drone was raised to height of c. 80m while the birds resettled. Forward flight was resumed at 80m and no further disturbance was observed. The photos were subsequently analysed and although a full count of sitting birds would be possible, the relatively high position of the drone during most of the flight meant that differentiation of species and nesting/roosting birds was difficult.
On the third and fourth trials the drone was launched and bought up to an altitude of 80m, moved into position then then slowly lowered, watching carefully for signs of disturbance. Over the course of 90 seconds the drone was lowered to 15m without any disturbance response. Photos were taken on the ‘auto’ setting and later analysed by zooming in on generic software (Microsoft Paint 3D) and the young of each species were counted. These results were then compared to the most recent standard boat-based productivity survey which had taken place just two days earlier. The drone survey revealed a surprising 64% increase in the number of black-headed gull Chroicocephalus ridibundus young recorded.
The analysis of the images via the method above took considerable time however and as a result (and due to the many other pressures on time during the breeding seabird season) further flights for the purpose of monitoring the rest of the colony did not take place during 2019. There are faster ways of analysing the images but these require some specialist software. It is also worth considering the possibility of engaging citizen scientists to analyse images e.g. SeabirdWatch.
Key Points when using drones
- Drone users must be suitably trained, CAA registered and compliant, and insured (RSPB drone users must follow the Code of Practice B9).
- Drone use must meet legal conditions and the flyer is responsible for ensuring these are met. A risk assessment should be in place including an assessment of any livestock present, obstructions, hazards, radio interference and disturbance-sensitive species present.
- Permissions must be in place to fly in the intended area. What is required will depend on the individual site but will include the landowner and if on a designated site, the relevant statutory agency (never fly within prohibited areas).
- Drones should only be used when weather conditions are suitable.
- A drone approach should be made at a height unlikely to disturb wildlife, 100-120m is recommended as a suitable approach height although in this case 80m above the tern and gull colony did not appear to cause disturbance. The drone can then be brought lower to record monitoring images.
- It will be necessary to calibrate between ground-based counts and drone-counts, as the methods have different detection levels (drones will not be better in every situation).
Tern chick diet monitoring method standardised for the little tern LIFE project
Tern chick provisioning is often monitored in academic studies as terns are surface feeders and so are more vulnerable to low food availability than diving species, making them good indicator species of food availability. Secondly, adults carry prey items (usually a single fish but occasionally multiple small fish) across their bill, making the prey visible to an observer, so monitoring methods are non-invasive. However, although he information derived can be very useful in understanding the drivers of tern productivity (Nisbet et al., 1995) and even longer-term population changes (Suddaby and Ratcliffe, 1997)., most nature reserves do not routinely monitor provisioning as it requires a significant investment of time.
As with all monitoring, it is important to first consider what questions will be asked of the data. Prey species composition is often important for conservationists seeking to understand changing marine systems. Prey species and size information and feeding frequency can together be used to calculate energy inputs and linked to fledging success.
Provisioning in terns is known to be affected by:
It is important to capture these data when setting up a provisioning monitoring project. Provisioning observations should either be spread evenly across daylight hours to provide a full picture of provisioning patterns or done at the same time of day on each occasion, to keep this constant. Similarly, observations should be made at different stages of the tide and across the breeding season (number and age of chicks in each observed nest must be known). Wind and sea conditions should be recorded as affecting foraging ability, temperature and rainfall may also affect adult behaviour (need for incubation) especially when chicks are young. Tide can be described in different ways, e.g. time since the last high tide (in which case also note the height of that tide), or high/falling/low/rising – if categories are used, clearly define these (does ‘high’ cover the period of two hours either side of the peak, or more, or less?). The observer name, site, tern species monitored, date, time and duration of the observation period should all be noted on the recording sheet.
Tern chick provisioning is often monitored in academic studies as terns are surface feeders and so are more vulnerable to low food availability than diving species, making them good indicator species of food availability. Secondly, adults carry prey items (usually a single fish but occasionally multiple small fish) across their bill, making the prey visible to an observer, so monitoring methods are non-invasive. However, although he information derived can be very useful in understanding the drivers of tern productivity (Nisbet et al., 1995) and even longer-term population changes (Suddaby and Ratcliffe, 1997)., most nature reserves do not routinely monitor provisioning as it requires a significant investment of time.
As with all monitoring, it is important to first consider what questions will be asked of the data. Prey species composition is often important for conservationists seeking to understand changing marine systems. Prey species and size information and feeding frequency can together be used to calculate energy inputs and linked to fledging success.
Provisioning in terns is known to be affected by:
- age of chicks – older chicks tend to receive larger prey items
- number of chicks – provisioning increases when there are more chicks (but not proportionately)
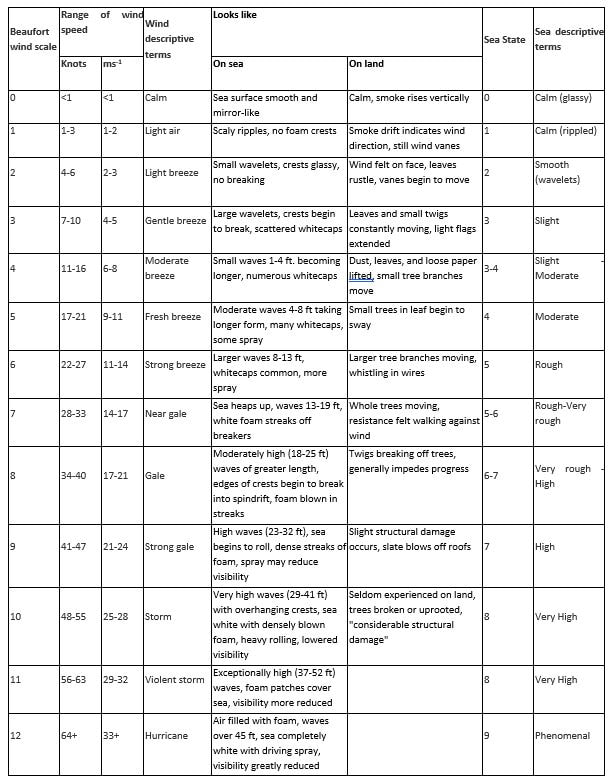
- wind speed – foraging efficiency increases with wind speed up to 6 knots, then declines
- sea conditions – foraging efficiency increases in slightly rough water, but when waters are too rough efficiency is reduced (see Appendix 2)
- time of day – provisioning activity tends to peak in the early morning and late afternoon
- state of tide – this has been found to be a factor in various studies, but different stages of the tide were used at different sites, so this may relate to local underwater topography
It is important to capture these data when setting up a provisioning monitoring project. Provisioning observations should either be spread evenly across daylight hours to provide a full picture of provisioning patterns or done at the same time of day on each occasion, to keep this constant. Similarly, observations should be made at different stages of the tide and across the breeding season (number and age of chicks in each observed nest must be known). Wind and sea conditions should be recorded as affecting foraging ability, temperature and rainfall may also affect adult behaviour (need for incubation) especially when chicks are young. Tide can be described in different ways, e.g. time since the last high tide (in which case also note the height of that tide), or high/falling/low/rising – if categories are used, clearly define these (does ‘high’ cover the period of two hours either side of the peak, or more, or less?). The observer name, site, tern species monitored, date, time and duration of the observation period should all be noted on the recording sheet.
Weather and tide data can be recorded from smartphone apps where there is reception. Ventusky and Windy are useful for wind speed and direction and for wave height. AyeTides and MyTideTimes are useful sources for tidal information. If multiple observers will be recording data from apps they should use the same ones and ensure that the same reference points are used and same data recorded. If multiple observers will be recording wind or wave data from environmental observations (see Appendix 2) they should, where possible, do some sessions together to calibrate how they assess conditions.
Provisioning observations themselves should consist of:
- prey species (or category e.g. sandeel, clupeid or gadoid)
- prey size – defined in terms of tern bill lengths, in increments of 0.5 bill lengths
- time of provisioning
- identity of nest
- outcome (was the fish eaten, dropped or kleptoparasitised (stolen)
Again, where possible, if a project includes multiple observers some effort should be made to calibrate observations by doing some joint observation sessions or looking at photographs together and agreeing size categories.
It should be possible to observe multiple tern nests simultaneously, but nests selected should be within one field of view to reduce the likelihood of missing provisioning events. The number of nests observed simultaneously will therefore depend on the density and topography within the colony, but in a dense colony 15-20 would be a useful sample size. Little terns Sternula albifrons will usually be more dispersed and therefore fewer nests can be observed. Marking the nests on a laminated A4 photograph or sketch map of the relevant part of the colony is a useful way to ensure consistency of observation, particularly if more than one person is recording data from the same nests.
The selection of nests for observation should also, where possible, take into account the adult ‘quality’. Older, more experienced and more productive birds tend to arrive at a colony first and select the best nest sites, often in the interior of a developing colony. Younger, less-experienced and later-arriving birds are more likely to nest on the periphery, these birds are more likely to fail and may provision their offspring at a reduced rate. However, the decision about the location of an observer needs to be pragmatic and cause minimum disruption to the colony. Often this will be in a hide or vantage point that cannot be moved, and the study nests will be limited to those which are easily observed from that point. It is therefore important to record the position of the study sites in relation to the whole colony.
Provisioning observations should be made for a minimum period of one hour, in order to make a meaningful comparison, but a 2 hour or longer period is preferable. The number of provisioning events at a sample of 20 nests may be quite small within an hour, especially early in the season when some eggs have not yet hatched, or late in the season when some birds have fledged.
Even the most skilled observer will miss a small proportion of provisioning events or be unable to identify a proportion of the prey items, often because of the direction of approach of the adult bird. There is also likely to be a certain amount of non-provisioning activity in the colony, as early on some pairs are still courting and unpaired birds may continue displaying with large fish until the middle of the breeding season. Fish chosen for display seem to be selected for their large size and are unlikely to be representative of the food resource generally. There is therefore limited value in recording the size and species of these fish, unless aiming to answer a question about displaying birds specifically.
The value of the provisioning data is greatly increased if the identity of the adults and/or chicks (or at least the brood), is known. This could mean that individual birds are caught and marked, by colour rings or other approved methods under licence from BTO. In some cases, it is possible to isolate ‘study nests’ within small enclosures, ensuring that provisioning happens within view and is of known individuals, however great care must be taken that nest enclosures do not compromise the survival of either chicks or adults. At colonies where enclosures are used to monitor productivity and chick condition, they could also be used for provisioning observations if the enclosures are in use and capable of being observed without additional disturbance.
Where individuals or broods can be identified, or where the study area is clearly defined the number and age of chicks should be recorded on each day when provisioning observations are made. Where adults are marked, it may also be useful to record which adult has brought the prey item. In the first days after hatching is likely that one adult will remain at the nest, but later in the breeding season, especially when there is little food available, both adults may be absent. However, recording detailed observations on the change-over of adults at the nest is often not useful and comes at the expense of observing a greater number of nests simultaneously. It is usually better to have a larger sample size of provisioning observations and less information about the movements of the adult birds.
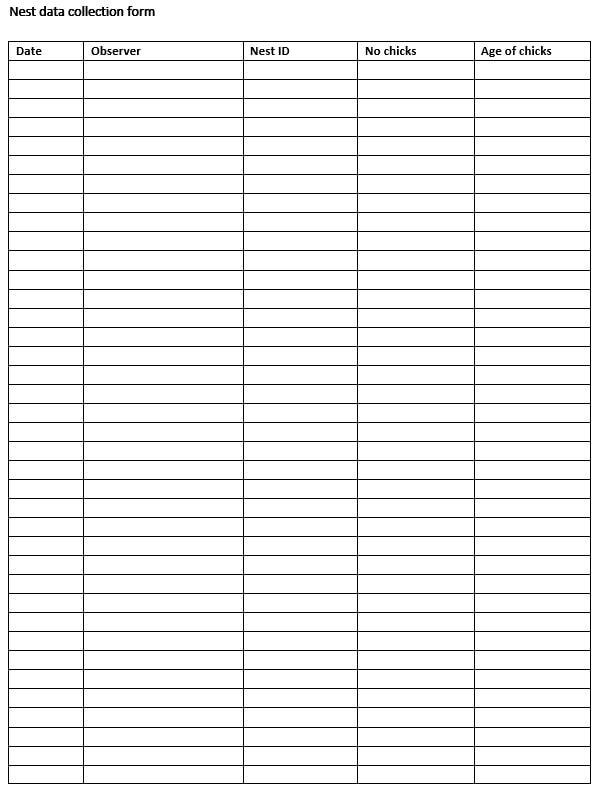
Examples of suitable data collection forms are provided in Appendix 1. Both the provisioning data form and the nest data form should be used, as chick age and number aids interpretation of the provisioning data. However, do not disturb the colony before immediately before the observation period, and in cold or wet conditions the colony should not be disturbed at all.
Use of motion-triggered cameras is frequently proposed as a method of reducing the labour-intensive nature of provisioning observation data collection. This may work for up to 3 days after hatching while chicks tend to stay on the nest site, but after this, unless nests are enclosed the chicks will wander and it is highly likely that some provisioning will take place off-camera. However, it may be possible to use high quality digital images taken by an observer to later allow checking of prey species and size. Gaglio et al (2016) used a DSLR and telephoto lens to photograph terns in flight returning to the colony with prey. This is a useful method for determining what prey items are being brought back to the colony but does not capture whether they are fed to chicks or not.
It is important to remember that these observations do not provide information about adult feeding, as adult terns are selective about which prey items are brought to their chicks and which they eat themselves. It is also important not to assume that all time spent away from the nest is time spent foraging, so the duration a bird is absent cannot be used for example to estimate distance travelled and therefore foraging area.
Prey species identification sheets
The Roseate Tern LIFE Project has produced prey ID sheets which can be downloaded from the project website and used to calibrate size observations and/or laminated for use in the field: Sandeel, Clupeidae, Gadiformes, and Other prey.
The Roseate Tern LIFE Project has produced prey ID sheets which can be downloaded from the project website and used to calibrate size observations and/or laminated for use in the field: Sandeel, Clupeidae, Gadiformes, and Other prey.
 Figure 4. Arctic tern chicks on the Farne Islands (M. Babcock)
Figure 4. Arctic tern chicks on the Farne Islands (M. Babcock)
Chick growth monitoring: a study from the Farne Islands
In addition to monitoring chick provisioning it is also possible to monitor chick growth and condition by taking repeated measurements of the chicks themselves. This requires repeated recapture of individual chicks, with associated disturbance, and is resource intensive as well as requiring the appropriate ringing licences. As a result, it is only done regularly at two colonies in the UK and Ireland: Rockabill and Lady’s Island Lake and occasionally elsewhere as part of PhD studies etc. The need to recapture the same chicks means that such measurements are most easily taken from ringed chicks in enclosures (see above).
Where food resources are limiting chicks usually maintain structural growth rates at the expense of gaining mass, so it is important to measure both chick weight and structural development such as wing length, head plus bill length etc. as it is the ratio between these that will provide information about development.
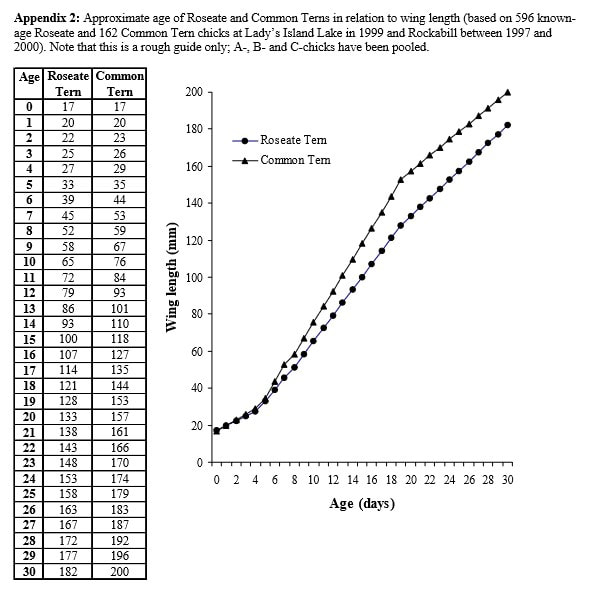
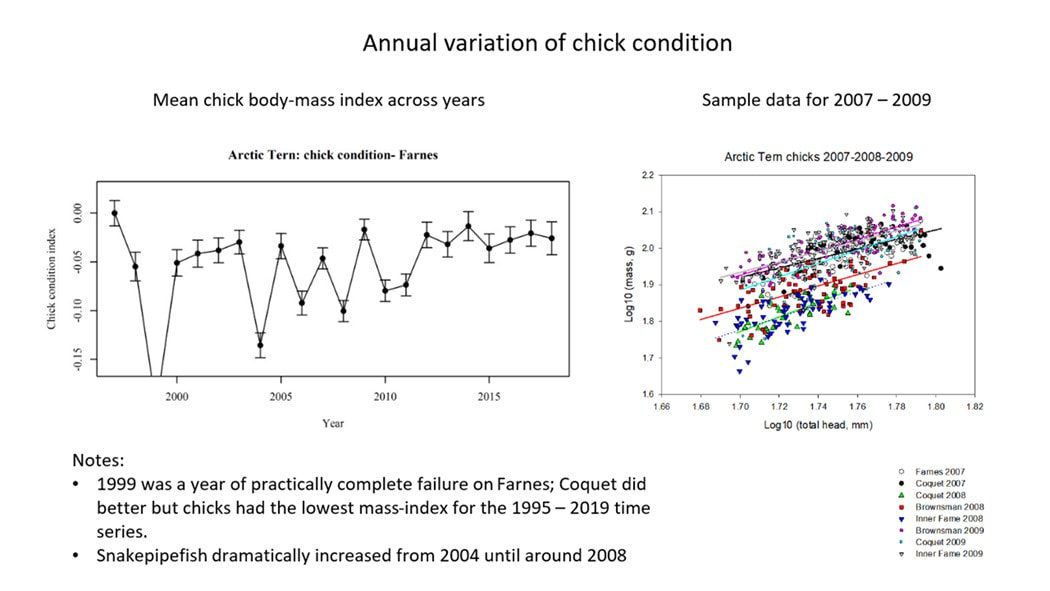
Measurements should usually be taken every few days as the chicks develop. Chick growth curves are sigmoidal (S shaped;(see graphs 1 and 2 below) but with a linear phase of growth from approximately four to 13 days. As a result, and as repeated measurements of the same subject are involved, statistical analysis of growth data between sites or over multiple years is complex.
To compare chick condition from year to year without the need for repeat measurements Dr Chris Redfern of Newcastle University developed an index of chick condition for Arctic terns Sterna paradisaea on the Farne Islands, which uses a single set of measurements of each chick during the linear growth phase. The index is based on measuring weight and head plus bill length.
He describes this method as follows:
“I established a size range (=age) from growth curves of known-age Arctic Tern chicks back in 1995 – 1998-ish, and on the basis of that I use chicks with total head lengths of ~49 mm (about 9 days after hatching in an Arctic tern, thus avoiding very young chicks) to ~61 mm (thus avoiding chicks that may have started a pre-fledging, post-asymptotic mass loss). Within that size range, plots of log mass versus log total head are linear and parallel between years. It relies on the structural measure (total head length) continuing at a similar rate regardless of nutrition (which is an approximation - in really bad years structural growth rate can be affected, but that just means that the condition index itself is not necessarily strictly linear with nutrition status [which is a caveat for year-to-year comparisons]). As a condition index, it wraps up skeletal, fat and muscle mass of the body together with meals currently sitting in the chicks proventriculus and the rest of its digestive system (so treat chicks gently so that they don’t regurgitate!). I think it is better to choose chicks across the specified range, rather than going for a narrower range of chick sizes.
The size range given above is for Arctic tern chicks, so ideally it would be useful to have ‘calibration’ data for total head length and mass for roseate and common tern chicks of known age, but we could extrapolate from the Arctic terns which are only a wee bit smaller (mean total head for adults would be around 71 mm), so for roseates (mean total head for adults of around 79 mm) going for chicks of total head length ~52 mm up to ~64 mm might be a reasonable approximation.
The measurements could be done in one session, or spread over two or more not too far apart (probably better, to avoid local temporal ‘spikes’ in food availability. Another option is to do the measurements at a similar date each year (perhaps determined by first egg date), representing the chicks from the first, main wave of nesting birds (avoiding late-comers and re-lays).”
For colonies where chicks are routinely ringed this method has the potential to enable year to year comparisons and comparisons between sites with less recording effort, and it would be useful to develop the method for other species.
In addition to monitoring chick provisioning it is also possible to monitor chick growth and condition by taking repeated measurements of the chicks themselves. This requires repeated recapture of individual chicks, with associated disturbance, and is resource intensive as well as requiring the appropriate ringing licences. As a result, it is only done regularly at two colonies in the UK and Ireland: Rockabill and Lady’s Island Lake and occasionally elsewhere as part of PhD studies etc. The need to recapture the same chicks means that such measurements are most easily taken from ringed chicks in enclosures (see above).
Where food resources are limiting chicks usually maintain structural growth rates at the expense of gaining mass, so it is important to measure both chick weight and structural development such as wing length, head plus bill length etc. as it is the ratio between these that will provide information about development.
Measurements should usually be taken every few days as the chicks develop. Chick growth curves are sigmoidal (S shaped;(see graphs 1 and 2 below) but with a linear phase of growth from approximately four to 13 days. As a result, and as repeated measurements of the same subject are involved, statistical analysis of growth data between sites or over multiple years is complex.
To compare chick condition from year to year without the need for repeat measurements Dr Chris Redfern of Newcastle University developed an index of chick condition for Arctic terns Sterna paradisaea on the Farne Islands, which uses a single set of measurements of each chick during the linear growth phase. The index is based on measuring weight and head plus bill length.
He describes this method as follows:
“I established a size range (=age) from growth curves of known-age Arctic Tern chicks back in 1995 – 1998-ish, and on the basis of that I use chicks with total head lengths of ~49 mm (about 9 days after hatching in an Arctic tern, thus avoiding very young chicks) to ~61 mm (thus avoiding chicks that may have started a pre-fledging, post-asymptotic mass loss). Within that size range, plots of log mass versus log total head are linear and parallel between years. It relies on the structural measure (total head length) continuing at a similar rate regardless of nutrition (which is an approximation - in really bad years structural growth rate can be affected, but that just means that the condition index itself is not necessarily strictly linear with nutrition status [which is a caveat for year-to-year comparisons]). As a condition index, it wraps up skeletal, fat and muscle mass of the body together with meals currently sitting in the chicks proventriculus and the rest of its digestive system (so treat chicks gently so that they don’t regurgitate!). I think it is better to choose chicks across the specified range, rather than going for a narrower range of chick sizes.
The size range given above is for Arctic tern chicks, so ideally it would be useful to have ‘calibration’ data for total head length and mass for roseate and common tern chicks of known age, but we could extrapolate from the Arctic terns which are only a wee bit smaller (mean total head for adults would be around 71 mm), so for roseates (mean total head for adults of around 79 mm) going for chicks of total head length ~52 mm up to ~64 mm might be a reasonable approximation.
The measurements could be done in one session, or spread over two or more not too far apart (probably better, to avoid local temporal ‘spikes’ in food availability. Another option is to do the measurements at a similar date each year (perhaps determined by first egg date), representing the chicks from the first, main wave of nesting birds (avoiding late-comers and re-lays).”
For colonies where chicks are routinely ringed this method has the potential to enable year to year comparisons and comparisons between sites with less recording effort, and it would be useful to develop the method for other species.
Predator monitoring methods
Much of the management that takes place at tern colonies is designed to deter predation. Without standardised monitoring, reports of predator activity can only be anecdotal. Systematic, timed observations of predator activity allow the quantification of predator activity across a season.
The key to monitoring anything that occurs in a patchy distribution (as predation attempts are likely to be sporadic, unless there is a really persistent predator targeting the tern colony) is to maximise the coverage, either by conducting long observations, or a great many shorter observations. It is useful to set a minimum observation period e.g. an hour, which should only begin when the colony has settled after any disturbance caused by the observer getting to the viewing point (although ideally disturbance should be avoided).
Just as tern provisioning is influenced by the time of day, so predator activity may follow a pattern, so observations should be spread across different times of day, tides, and times of the season. Weather conditions may also be relevant and should be recorded. Whether observations cover the whole or part of the colony will depend on the colony size and visibility. The number of tern nests and the age of any chicks and the response of adult birds should be included. The predator species and outcome of any predation attempts should be recorded. If multiple observers are involved it would be useful to agree what constitutes a predation attempt to ensure a consistent approach.
If the intention is to accurately quantify predator activity at the colony, do not drive away predators during the timed watches.
Much of the management that takes place at tern colonies is designed to deter predation. Without standardised monitoring, reports of predator activity can only be anecdotal. Systematic, timed observations of predator activity allow the quantification of predator activity across a season.
The key to monitoring anything that occurs in a patchy distribution (as predation attempts are likely to be sporadic, unless there is a really persistent predator targeting the tern colony) is to maximise the coverage, either by conducting long observations, or a great many shorter observations. It is useful to set a minimum observation period e.g. an hour, which should only begin when the colony has settled after any disturbance caused by the observer getting to the viewing point (although ideally disturbance should be avoided).
Just as tern provisioning is influenced by the time of day, so predator activity may follow a pattern, so observations should be spread across different times of day, tides, and times of the season. Weather conditions may also be relevant and should be recorded. Whether observations cover the whole or part of the colony will depend on the colony size and visibility. The number of tern nests and the age of any chicks and the response of adult birds should be included. The predator species and outcome of any predation attempts should be recorded. If multiple observers are involved it would be useful to agree what constitutes a predation attempt to ensure a consistent approach.
If the intention is to accurately quantify predator activity at the colony, do not drive away predators during the timed watches.
Predator monitoring by camera
Motion activated trap cameras can be extremely useful in monitoring predator activity, especially at night. Common brands in use at tern colonies include Bushnell, Browning and LtL-Acorn and there was a discussion on this subject on the Little Tern Forum in early summer 2020. Consider what you need the camera to do (e.g. will it be used in the day or at night, where will it be positioned) and check the specification of the individual model.
The detection zone of the sensor is the main determinant in how many images are captured, and this has two components: distance and angle. Some cameras have a detection zone angle wider than the field of view on the lens, which can make it more likely to capture fast moving animals, but can also produce empty photos when animals enter the detection zone but don't quite make into the view of the camera's lens. Conversely, trail cameras with narrow detection zones almost always produce perfectly centred pictures with very few empty frames. However, these also fail to detect a substantial number of animals that wander into the view of the camera lens, but don't make it to the narrow detection zone.
Trigger speed is the delay between a detection and a picture being recorded. A slow trigger speed won't work well looking across a track or path, as the animal is likely to have passed by the time a picture is taken, but if looking along the route of the animal it won’t matter.
Recovery Time is probably the least talked about feature of Trail Cameras, but one of the most important, as it is the time it takes a camera to be triggered, take a picture, store that picture and be ready for the next picture. Some cameras take 60 seconds while some trail cameras recover instantly. This is less of an issue if capturing video rather than still images.
Camera traps can either be placed on specific nests, though it is important not to draw the predator’s attention to a nest location by positioning a camera near it (corvids are particularly good at working this out). Cameras on nests will catch predation of eggs or young chicks, but after a few days older chicks are likely to be mobile and not always in front of the camera. An alternative is to position a camera on likely access routes predators might use to access the colony. Or a camera can be used to confirm the presence of a specific predator at sites where signs have been found, as in the case of the otter Lutra lutra on Coquet Island.
Cameras will require batteries and SD cards to be replaced so should be placed where they can be accessed with minimal disturbance. Camera traps produce a LOT of images, so any plan to monitor with trap cameras needs to include time to sift through them.
Motion activated trap cameras can be extremely useful in monitoring predator activity, especially at night. Common brands in use at tern colonies include Bushnell, Browning and LtL-Acorn and there was a discussion on this subject on the Little Tern Forum in early summer 2020. Consider what you need the camera to do (e.g. will it be used in the day or at night, where will it be positioned) and check the specification of the individual model.
The detection zone of the sensor is the main determinant in how many images are captured, and this has two components: distance and angle. Some cameras have a detection zone angle wider than the field of view on the lens, which can make it more likely to capture fast moving animals, but can also produce empty photos when animals enter the detection zone but don't quite make into the view of the camera's lens. Conversely, trail cameras with narrow detection zones almost always produce perfectly centred pictures with very few empty frames. However, these also fail to detect a substantial number of animals that wander into the view of the camera lens, but don't make it to the narrow detection zone.
Trigger speed is the delay between a detection and a picture being recorded. A slow trigger speed won't work well looking across a track or path, as the animal is likely to have passed by the time a picture is taken, but if looking along the route of the animal it won’t matter.
Recovery Time is probably the least talked about feature of Trail Cameras, but one of the most important, as it is the time it takes a camera to be triggered, take a picture, store that picture and be ready for the next picture. Some cameras take 60 seconds while some trail cameras recover instantly. This is less of an issue if capturing video rather than still images.
Camera traps can either be placed on specific nests, though it is important not to draw the predator’s attention to a nest location by positioning a camera near it (corvids are particularly good at working this out). Cameras on nests will catch predation of eggs or young chicks, but after a few days older chicks are likely to be mobile and not always in front of the camera. An alternative is to position a camera on likely access routes predators might use to access the colony. Or a camera can be used to confirm the presence of a specific predator at sites where signs have been found, as in the case of the otter Lutra lutra on Coquet Island.
Cameras will require batteries and SD cards to be replaced so should be placed where they can be accessed with minimal disturbance. Camera traps produce a LOT of images, so any plan to monitor with trap cameras needs to include time to sift through them.
Ringing and colour ringing projects
Ringing tern chicks and/or adults with metal or with field readable plastic rings is outside the scope of this guidance since it requires specialist training in accordance with the relevant ringing scheme. But, wherever possible, efforts should be made to read rings and report any ringed birds which are present in a colony.
At the Gronant little tern colony in North Wales Go-Pro cameras have been used to supplement colour ring reading efforts. Sample nests were chosen and monitored with video footage and camera stills taken at 1 second intervals, with the camera one to two feet away from nests. Twenty-five colour ring readings were collected from 92 nests checked.
Ringing tern chicks and/or adults with metal or with field readable plastic rings is outside the scope of this guidance since it requires specialist training in accordance with the relevant ringing scheme. But, wherever possible, efforts should be made to read rings and report any ringed birds which are present in a colony.
At the Gronant little tern colony in North Wales Go-Pro cameras have been used to supplement colour ring reading efforts. Sample nests were chosen and monitored with video footage and camera stills taken at 1 second intervals, with the camera one to two feet away from nests. Twenty-five colour ring readings were collected from 92 nests checked.
Biosecurity Monitoring: Green Island, Northern Ireland case study
Green Island in Carlingford Lough is owned by the National Trust and Managed by the RSPB. It hosts a significant mixed breeding colony of terns and gulls as well as some waders and is a grey and common seal haul out. It is a small island (0.15 ha) assessed in the Biosecurity Plan as being at moderate risk of invasion by mammalian invasive non-native species (INNS) as it is only around 600 m from the shore at mean high water with some rocky outcrops between the island and the shore which could act as potential ‘stepping stones’, although the tidal streams are strong. The pathways by which mammalian predators could reach Green Island vary: house mice are most likely to reach the island as stowaways by boat, in equipment or personal belongings (low likelihood), while brown rats (medium likelihood), American mink and otters (high likelihood) are more likely to swim (Wojcieszek et al. 2019).
Staff usually visit Green Island around 15 times between April and July for monitoring and 2-3 times between August and March for habitat management. Barriers to invasion are created by ways of working such as packing (or repacking) bags on the day of travel, checking any materials transported onto the island, and moving only small volumes of materials at one time.
Routine monitoring takes place on every visit, checking four bait stations which contain non-toxic wax monitoring blocks, making visual searches for rat, mink and otter signs (droppings, footprints, feeding signs, corpses). There are procedures in place in case either suspected or definite signs are found. For signs which are possible (but not probable or definite) the intensive monitoring protocol will be initiated, including additional non-toxic monitoring such as tracking tunnels, UV light detection, or trail cameras as well as wax monitoring blocks and traps. There is also an incursion response plan for probable or definite signs of mice, rats, mink and otters (a European Protected Species), each of which requires a different approach as detailed in the biosecurity plan.
Green Island in Carlingford Lough is owned by the National Trust and Managed by the RSPB. It hosts a significant mixed breeding colony of terns and gulls as well as some waders and is a grey and common seal haul out. It is a small island (0.15 ha) assessed in the Biosecurity Plan as being at moderate risk of invasion by mammalian invasive non-native species (INNS) as it is only around 600 m from the shore at mean high water with some rocky outcrops between the island and the shore which could act as potential ‘stepping stones’, although the tidal streams are strong. The pathways by which mammalian predators could reach Green Island vary: house mice are most likely to reach the island as stowaways by boat, in equipment or personal belongings (low likelihood), while brown rats (medium likelihood), American mink and otters (high likelihood) are more likely to swim (Wojcieszek et al. 2019).
Staff usually visit Green Island around 15 times between April and July for monitoring and 2-3 times between August and March for habitat management. Barriers to invasion are created by ways of working such as packing (or repacking) bags on the day of travel, checking any materials transported onto the island, and moving only small volumes of materials at one time.
Routine monitoring takes place on every visit, checking four bait stations which contain non-toxic wax monitoring blocks, making visual searches for rat, mink and otter signs (droppings, footprints, feeding signs, corpses). There are procedures in place in case either suspected or definite signs are found. For signs which are possible (but not probable or definite) the intensive monitoring protocol will be initiated, including additional non-toxic monitoring such as tracking tunnels, UV light detection, or trail cameras as well as wax monitoring blocks and traps. There is also an incursion response plan for probable or definite signs of mice, rats, mink and otters (a European Protected Species), each of which requires a different approach as detailed in the biosecurity plan.
References
Coquet Island - Monitoring Guidelines (Revised 2017). Unpublished RSPB internal document.
Gaglio, D., Cook, T.R., Connan, M., Ryan, P.G. and Sherley, R.B. (2017), Dietary studies in birds: testing a non‐invasive method using digital photography in seabirds. Methods in Ecology and Evolution, 8: 214-222. doi:10.1111/2041-210X.12643
Newton, S.F. & Glenister, L.J. (2008). Rockabill Tern Manual (revised edition, November 2008). Unpublished BirdWatch Ireland Report, Newtownmountkennedy, Co. Wicklow.
Nisbet, I.C.T., Spendelow, J.A. and Hatfield, J.S. (1995) 'Variations in Growth of Roseate Tern Chicks', The Condor, 97(2), pp. 335-344.
Suddaby, D. and Ratcliffe, N. (1997) 'The Effects of Fluctuating Food Availability on Breeding Arctic Terns (Sterna paradisaea)', The Auk, 114(3), pp. 524-530.
Walsh, P.M., Halley, D.J., Harris, M.P., del Nevo, A., Sim, I.M.W. & Tasker, M.L. (1995). Seabird monitoring handbook for Britain and Ireland. JNCC / RSPB / ITE / Seabird Group, Peterborough. ISBN 1 873701 73 X
Wojcieszek M., Tickner M. and Varnham K. (2019) Biosecurity Plan for RSPB Green Island, Carlingford Lough. RSPB unpublished internal document.
Acknowledgements
Thanks to: Paul Morrison (RSPB Coquet Island), Chris Redfern (Newcastle University), Daniel Piec (RSPB Roseate Tern LIFE Project), Steve Newton (Birdwatch Ireland).
Coquet Island - Monitoring Guidelines (Revised 2017). Unpublished RSPB internal document.
Gaglio, D., Cook, T.R., Connan, M., Ryan, P.G. and Sherley, R.B. (2017), Dietary studies in birds: testing a non‐invasive method using digital photography in seabirds. Methods in Ecology and Evolution, 8: 214-222. doi:10.1111/2041-210X.12643
Newton, S.F. & Glenister, L.J. (2008). Rockabill Tern Manual (revised edition, November 2008). Unpublished BirdWatch Ireland Report, Newtownmountkennedy, Co. Wicklow.
Nisbet, I.C.T., Spendelow, J.A. and Hatfield, J.S. (1995) 'Variations in Growth of Roseate Tern Chicks', The Condor, 97(2), pp. 335-344.
Suddaby, D. and Ratcliffe, N. (1997) 'The Effects of Fluctuating Food Availability on Breeding Arctic Terns (Sterna paradisaea)', The Auk, 114(3), pp. 524-530.
Walsh, P.M., Halley, D.J., Harris, M.P., del Nevo, A., Sim, I.M.W. & Tasker, M.L. (1995). Seabird monitoring handbook for Britain and Ireland. JNCC / RSPB / ITE / Seabird Group, Peterborough. ISBN 1 873701 73 X
Wojcieszek M., Tickner M. and Varnham K. (2019) Biosecurity Plan for RSPB Green Island, Carlingford Lough. RSPB unpublished internal document.
Acknowledgements
Thanks to: Paul Morrison (RSPB Coquet Island), Chris Redfern (Newcastle University), Daniel Piec (RSPB Roseate Tern LIFE Project), Steve Newton (Birdwatch Ireland).
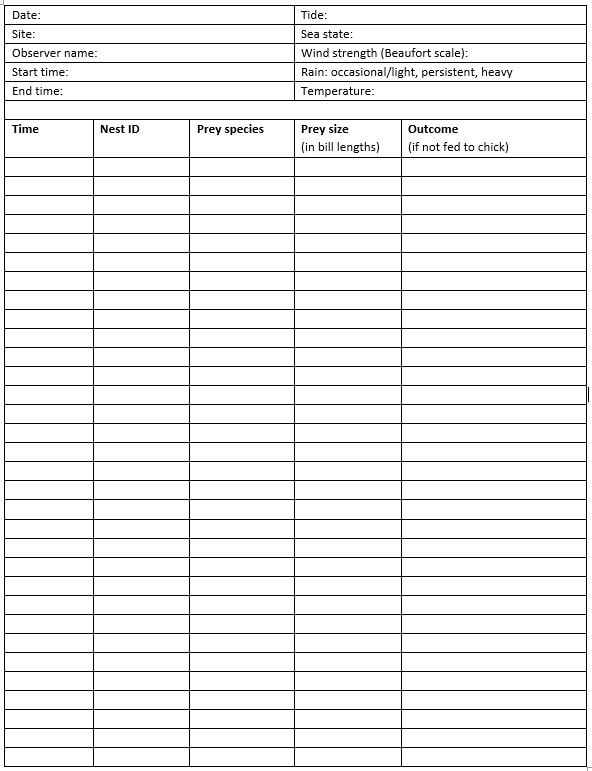
Appendix 1. Provisioning data collection form
Appendix 2. Weather and sea state descriptions